Table of Contents
The origin of Polystyrene
Polystyrene, also known as Polystyrol, is a widely used polymer produced through the polymerization of styrene.
It is a transparent, rigid, and brittle material that comes in various forms, such as Expanded Polystyrene (EPS) and Extruded Polystyrene (XPS). Polystyrene is commonly used in packaging, insulation, containers, and other everyday products.
It is water-resistant, chemically inert, and resistant to acids and bases but sensitive to organic solvents. Commercial production of Polystyrene began in the 1930s and is manufactured and processed worldwide.
EPS, a form of Polystyrene, is frequently used in packaging, insulation, and construction.

The crystallinity of PS
Polystyrene exists in two main forms: atactic and syndiotactic Polystyrene.
Atactic Polystyrene is amorphous, meaning it lacks a regular, ordered structure and therefore exhibits no crystallinity.
Syndiotactic Polystyrene, on the other hand, has a highly regular and ordered structure, with phenyl groups alternating on the linear carbon backbone. This regularity allows the molecules to easily pack into crystals, resulting in the formation of a crystalline structure.
The crystallinity of syndiotactic Polystyrene leads to a higher melting temperature and greater rigidity compared to atactic Polystyrene.
However, it is important to note that no polymer is entirely crystalline, as a fully crystalline polymer would be too brittle for use as a plastic. The presence of amorphous regions in polymers, including Polystyrene, imparts toughness, allowing the polymer to bend without breaking and absorb energy.

The melting point of Polystyrene
Polystyrene is a thermoplastic polymer that typically exists in a solid state at room temperature but melts when heated for shaping or extrusion.
The melting point of Polystyrene varies depending on its form and structure. Isotactic Polystyrene, which has a regular structure, has a melting temperature of about 240°C, while syndiotactic Polystyrene, which also has a regular structure, has a slightly higher melting temperature of around 270°C. This melting point can be measured using a Dynamic Differential Scanning Calorimeter (DSC), which allows for precise determination of the melting temperature and other thermal properties of materials.
Amorphous Polystyrene, which lacks a regular structure, does not melt at a specific temperature but gradually softens around 100°C, known as the glass transition temperature. These differences in melting temperature are related to the molecular arrangement and crystallinity of Polystyrene.
Pure solid Polystyrene is colorless, hard, and has limited flexibility. It can be molded into shapes with fine details and can be transparent or manufactured in various colors. It is economical and is used in the production of plastic model kits, license plate frames, plastic cutlery, CD jewel cases, model making, as an alternative material for records, and many other items where a fairly rigid, cost-effective plastic is desired.
Polystyrene can exist in a solid or foamed form. General Polystyrene is clear, hard, and brittle. It is an inexpensive resin per weight unit and has a relatively low melting point. It is one of the most widely used plastics, with production amounting to several million tons annually.

The melting point of Polystyrene
The thermal stability of Polystyrene depends on its structure and composition. Pure Polystyrene is not stable at high temperatures and tends to undergo thermal decomposition.
However, thermal stability can be improved by adding stabilizers. Isotactic Polystyrene, which has a regular structure, is typically more thermally stable than amorphous Polystyrene.
The thermal stability of Polystyrene can be measured using various methods, including a Chip-DSC (DSC) or through thermogravimetric analysis (TGA) using a STA L82 .
The DSC method measures the amount of heat absorbed or released by a material during a controlled temperature increase, allowing the determination of melting and softening points as well as the investigation of phase transitions and reactions. The TGA method measures the weight change of a material as a function of temperature and can be used to determine the thermal stability and decomposition temperatures of materials.

The glass transition temperature of Polystyrene
The Glass Transition Temperature (Tg) of Polystyrene typically occurs around 100 °C. This temperature marks the transition of the amorphous polymer from a hard, glassy state to a soft, rubbery state.
The Tg can be measured using a Chip-DSC, which measures the amount of heat absorbed or released by a material during a controlled temperature increase, allowing the determination of melting and softening points as well as the investigation of phase transitions and reactions.
The Glass Transition Temperature is an important parameter that affects the mechanical properties and processability of Polystyrene. Above the Tg, Polystyrene becomes soft and malleable, making it suitable for processing techniques like injection molding, while below the Tg, it becomes hard and brittle.

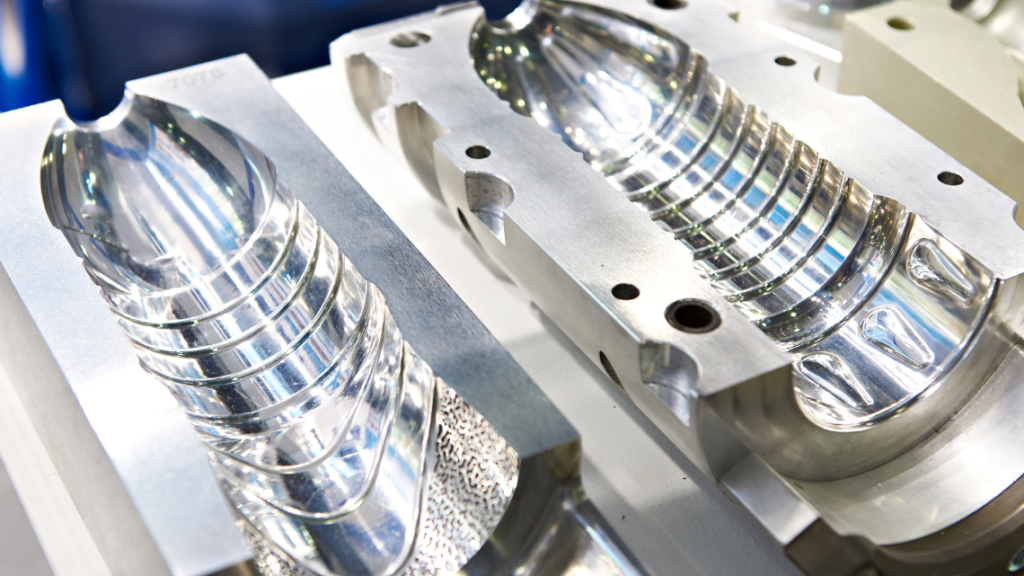
The production of Polystyrene parts by injection moulding
The production of Polystyrene parts through injection molding is a common process where molten Polystyrene is injected into a mold to achieve the desired shape. This process requires specialized injection molding machines and molds, typically made of steel or aluminum.
Polystyrene is well-suited for injection molding due to its good flowability and formability. The strength and formability of the material can be measured using a DIL L75 or a TMA. The DIL L75 is a dilatometer that measures the change in length of a material as a function of temperature, allowing insights into the thermal expansion and contraction of the material.
A Thermomechanical Analysis (TMA) measures the change in length of a material as a function of temperature and the force applied to the material, enabling the determination of glass transition and softening temperatures, as well as the investigation of changes in shape and stresses within the material.
Different types of PS
Polystyrene is a versatile polymer used in various forms and applications.
Some of the different types of Polystyrene include:
Solid Polystyrene:
- Is transparent, hard, brittle, and moderately rigid in its unaltered state. It is used for making plastic model kits, license plate frames, plastic cutlery, and CD jewel cases.

Expanded Polystyrene (EPS):

- Used as foam insulation in construction. It is also used in granular form for the production of polystyrene concrete, which is used in the construction industry as a building material.
Polystyrene Film:
- Used in film production and is transparent, durable, and printable.

Application: PS granules
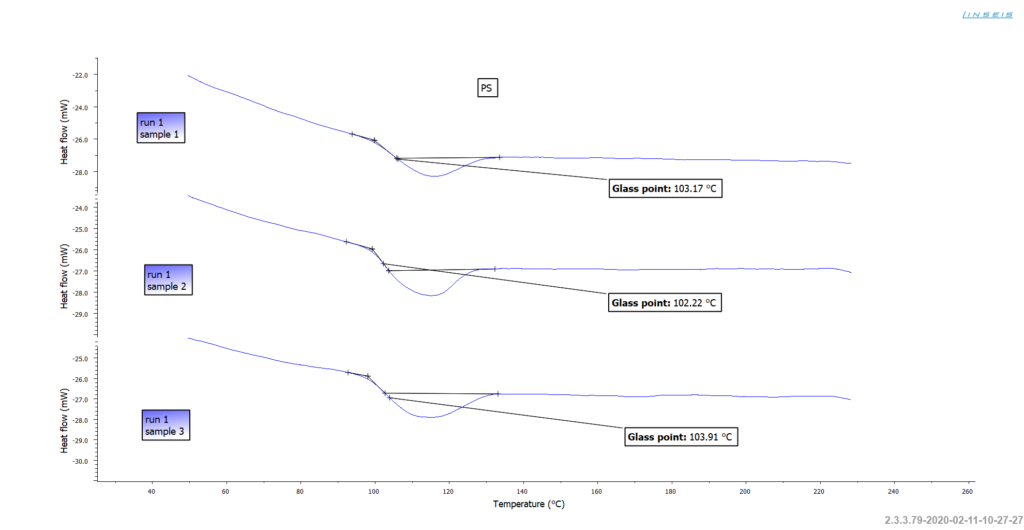
DEVICE | CHIP-DSC 1 (Chip-DSC L66 Basic) |
|---|---|
| Heating rate | 50 K/minute |
| Sample mass | approx. 15 mg |
| Sample tray | Open aluminium pans |
| Gas | Static air |
Environmental impact of Polystyrene
Polystyrene, especially expanded polystyrene (EPS), has significant environmental impacts. The production of polystyrene requires the use of petroleum, leading to a significant climate and environmental footprint.
After use, polystyrene, as a major component, contributes to terrestrial and marine waste. It is a common element in coastal debris and can persist in the environment for a long time as it degrades very slowly.
Additionally, polystyrene can leach chemicals in landfills, leading to further environmental issues. Furthermore, polystyrene poses a threat to the health of both humans and animals as it breaks down into small particles that can be ingested by wildlife.
Styrene, a major component of polystyrene, can also cause liver damage and damage to nerve tissues. Due to these environmental impacts, the use of polystyrene is already restricted or banned in some regions.

