Oxidation
Table of Contents
Oxidation is a chemical process that takes place when electrons are lost from a molecule, atom, or ion. In the context of calorimetry, oxidation is often studied as the thermal analysis, where it refers to the burning or breaking down of substances, to release energy in the form of heat.
Differential scanning calorimetry (DSC) is a technique that can be used to characterize this process, as it measures the heat flow associated with the oxidation of a sample as it is heated or cooled. The principle behind measuring oxidation using DSC (Differential Scanning Calorimetry) is based on the exothermic nature of oxidation reactions.
In DSC, oxidation can be studied isothermally or non-isothermally. For isothermal oxidation studies, the DSC crucible temperature is set at a constant temperature and the oxidation of the sample is monitored over time.
The onset of oxidation is typically indicated by the appearance of an exothermic peak in the DSC curve, which corresponds to the release of heat as the sample oxidizes. The time at which the exothermic peak appears is known as the oxidation induction time (OIT) and is a measure of the resistance of the sample to oxidation. The method is illustrated in Fig. 1.
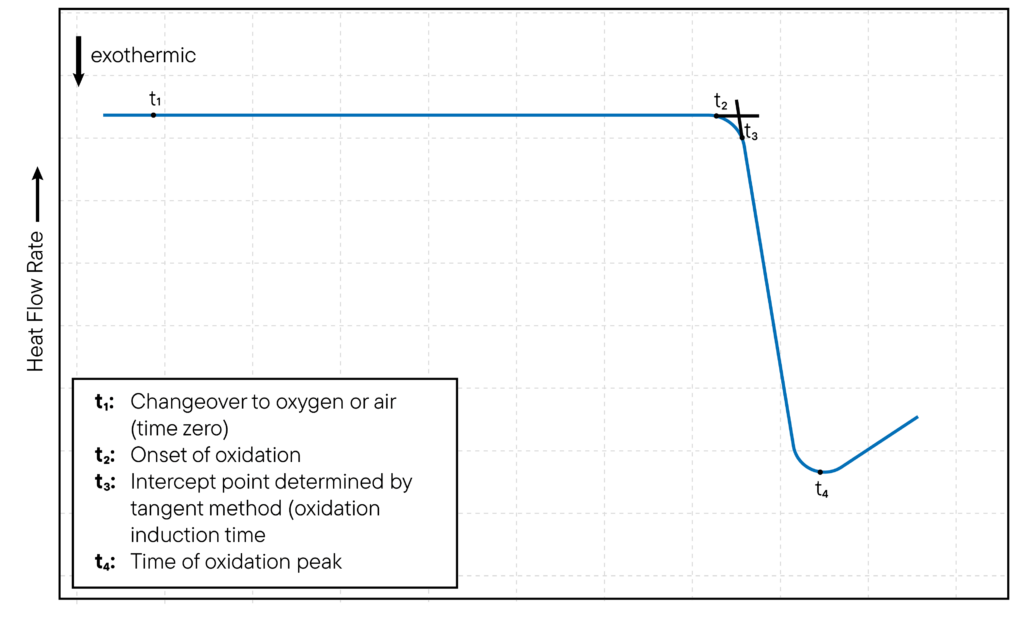
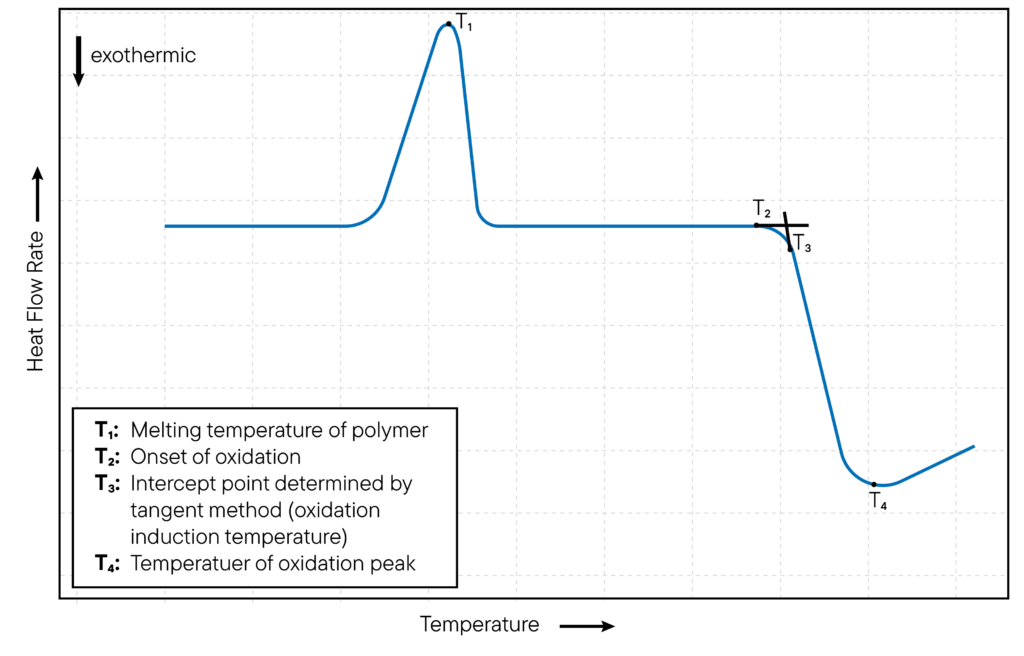
For non-isothermal oxidation studies, the DSC cell temperature is increased at a constant rate and the oxidation of the sample is monitored as a function of temperature.
The DSC instrument detects an exothermic peak in the DSC curve, at the onset when a sample undergoes oxidation and releases heat. The temperature at which the peak appears is known as the oxidation onset temperature (OOT).
The method is illustrated in Fig. 2. The OOT is a measure of the thermal stability of the sample and is used to assess the oxidative stability of materials.
In summary, by measuring the oxidation induction time (OIT) or the oxidation onset temperature (OOT) at different temperatures, the activation energy for oxidation can be determined, which provides insight into the thermal stability of the sample.
DSC can also be used to assess the effects of antioxidants and other additives on the oxidative stability of sample, which is important for their quality and shelf life.
DSC can also be used to determine the activation energy for oxidation, which is a measure of the energy required to initiate the oxidation process.
The activation energy can be calculated using the Arrhenius equation, which relates the rate of a chemical reaction to the temperature at which it occurs. The activation energy for oxidation can be determined by measuring the oxidation induction time (OIT) or the oxidation onset temperature (OOT) at different temperatures and plotting the data on an Arrhenius plot.
The slope of the resulting line is used to calculate the activation energy for oxidation. The DSC curve can be analysed to determine several parameters related to oxidation, such as onset temperature, peak temperature, and heat of oxidation.
Both OIT and OOT are important parameters in assessing the oxidative stability of materials, and antioxidant effectiveness can be compared particularly for polymers.
A longer OIT or higher OOT indicates greater oxidative stability and resistance to degradation. Factors that can affect the accuracy of DSC measurements for oxidation include the sample preparation, instrumentation, and experimental conditions, such as the heating rate and atmosphere.
Thermal analysis can be utilized to investigate the oxidation of metals in an oxygen-containing atmosphere, where a metal oxide is formed and the temperature- or time-dependent mass increase can be observed using techniques such as TGA.
Decomposition during oxidation
Decomposition refers to the breakdown of a compound into smaller molecules or elements [1, section 3.4.4]. If no oxidant such as atmospheric oxygen is present during a measuement, pyrolysis starts at a substance-dependent temperature. The substance system is split by the input of heat and decomposes. In a DSC, this can be implemented by using an inert sample gas such as nitrogen, as otherwise oxidation can occur, which affects this process. Decomposition is an endothermic process.
Citations:
- Stephen M. Hsua and Chun-I Chenb, A chemical kinetics model to predict diesel engine performance.Part II. Bench-test procedures: Tribology Letters, Vol. 14, No. 2, February 2003.
- B. Wunderlich, Thermal Analysis of Polymeric Materials. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg, 2005.
- G. W. Ehrenstein, G. Riedel und P. Trawiel, Thermal analysis of plastics: Theory and practice. Munich: Hanser, 2004.
- Plastics – Differential Scanning Calorimetry (DSC), ISO 11357-6, 2008.
- Mahbuba Islam, Anna Kaczmarek, and Jolanta Tomaszewska-Gras, Differential scanning calorimetry as a tool to assess the oxidation state of cold-pressed oils during shelf-life: Journal of Food Measurement and Characterization (2023) 17:6639–6651.
