Table of Contents
What is the Difference Between Endothermic and Exothermic Reactions?
The main difference between exothermic and endothermic reactions lies in the heat exchange with the surroundings:
- Endothermic reactions absorb energy in the form of heat from their surroundings, which leads to the surroundings becoming colder. This means that the products have more energy than the reactants, and the reaction requires heat, leading to a positive change in enthalpy (ΔH).
- Exothermic reactions release energy in the form of heat to their surroundings, which leads to the surroundings becoming warmer. In this case, the products have less energy than the reactants, and the reaction releases heat, leading to a negative change in enthalpy (ΔH).
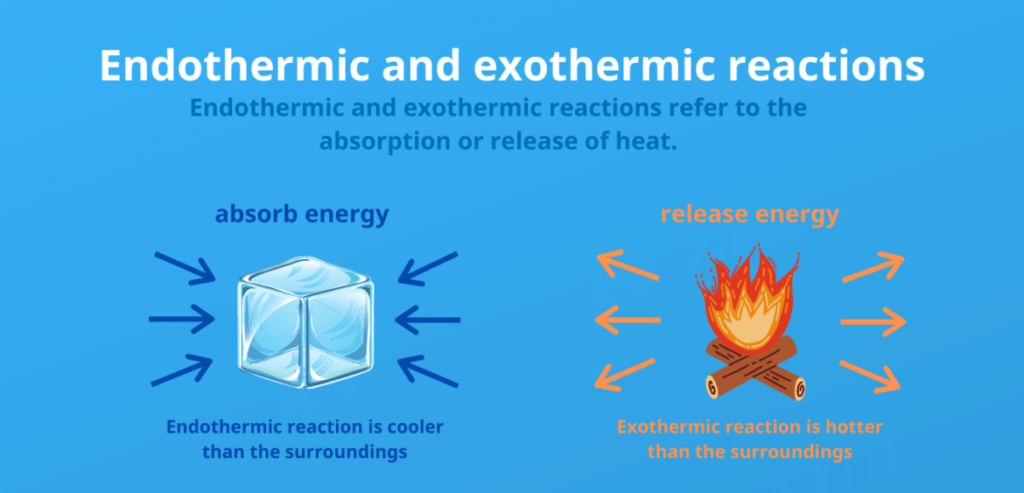
How to Identify an Exothermic or Endothermic Reaction?
There are two methods to recognize exothermic reactions:
- Temperature Change:
- In an exothermic reaction, energy is released, leading to an increase in the temperature of the reaction mixture. In contrast, in an endothermic reaction, energy is absorbed, resulting in a decrease in temperature. You can observe changes in temperature by placing a thermometer in the reaction mixture.
- Enthalpy Change:
- The change in enthalpy (ΔH) is the difference between the energy of the products and the energy of the reactants. If ΔH is negative, it indicates an exothermic chemical reaction, because more energy is released when the products are formed than is used to break down the reactants. If ΔH is positive, it indicates an endothermic chemical reaction, because less energy is released when the products are formed than the energy used to break down the reactants.
Exothermic and Endothermic Reactions Have Different Effects on the Environment:
Exothermic reactions release heat into the environment, which can have both positive and negative environmental impacts. They are involved in processes such as respiration, combustion, and energy production. However, exothermic reactions can also contribute to environmental problems like air and water pollution.
Further Examples of Exothermic Reactions:
- Combustion of Wood or Coal:
- The combustion of wood or coal releases heat, making it an exothermic reaction. These reactions are used in heaters, furnaces, and engines for energy production.
- Oxidation of Metals:
- When metals react with oxygen, like iron forming rust, it is an exothermic reaction where heat is released.
- Chemical Explosives:
- Explosions in chemical explosives are exothermic reactions that release large amounts of energy in the form of heat and pressure.
Endothermic reactions absorb heat from the surroundings. Although they are essential for processes like photosynthesis and cooking, they generally have a lesser direct impact on the environment compared to exothermic reactions.
Further Examples of Endothermic Reactions:
- Photosynthesis:
- In photosynthesis, plants absorb carbon dioxide from the air and convert it using light energy into sugar and oxygen. This is an endothermic reaction as energy in the form of light is absorbed.
- Boiling Water:
- Heating water to bring it to a boil is an endothermic reaction. During the boiling process, energy in the form of heat is supplied to convert the water particles into steam.
- Dissolving Salt in Water:
- When salt is dissolved in water, the salt molecules absorb heat from the surroundings to break down the crystals and disperse in a dissolved form.
