Simultaneous Thermal Analysis
Simultaneous Thermal Analysis – Thermogravimetry & Differential Scanning Caloriemtry
Simultaneous Thermal Analysis (STA) allows the investigation of Thermogravimetric (TG) effects coupled with the recording of a Differential Scanning Calorimetry (DSC) heat flow signal.
The analysis methods TG and DTA/DSC are applied simultaneously to the same sample. The results of both measuring methods can be compared directly. The influence of changed sample atmospheres on the reaction equilibrium, as can occur with individual measurements, is excluded.
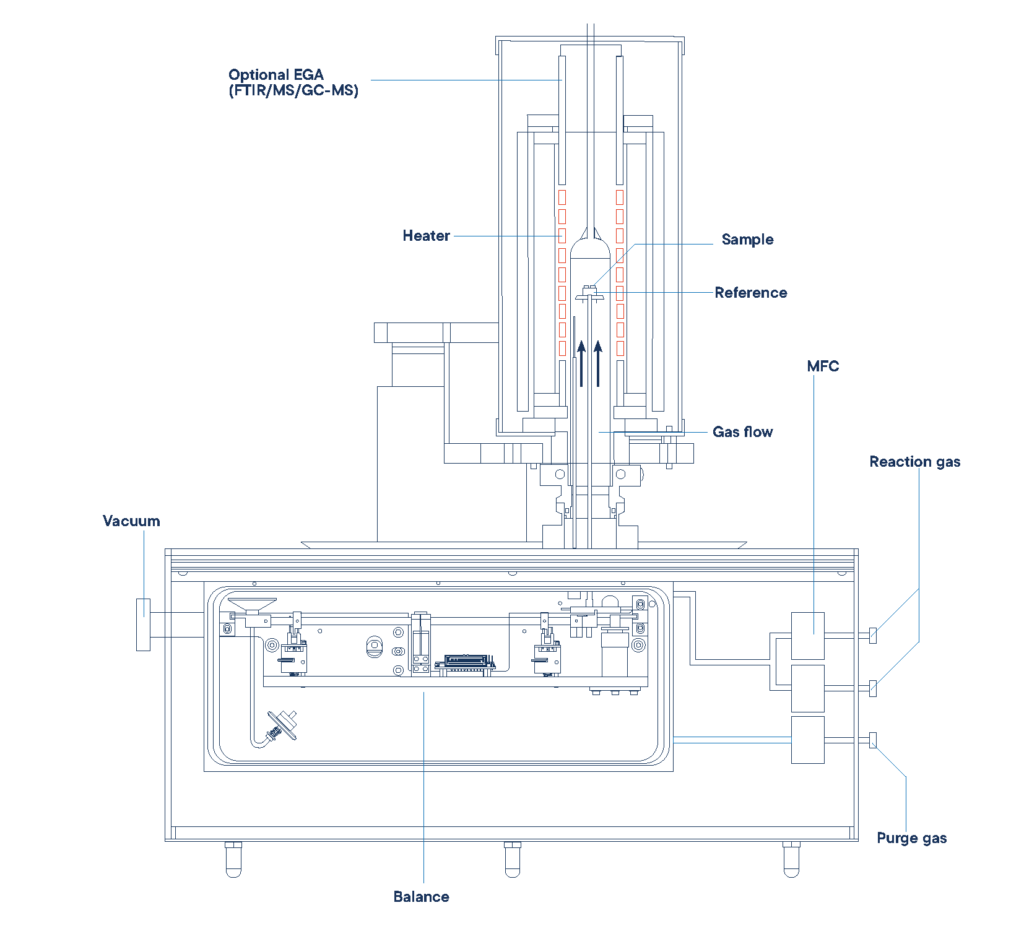
Setup of an STA measurement (simultaneous thermal analysis). DSC and TGA values are determined during the measurement.
Example of a measurement
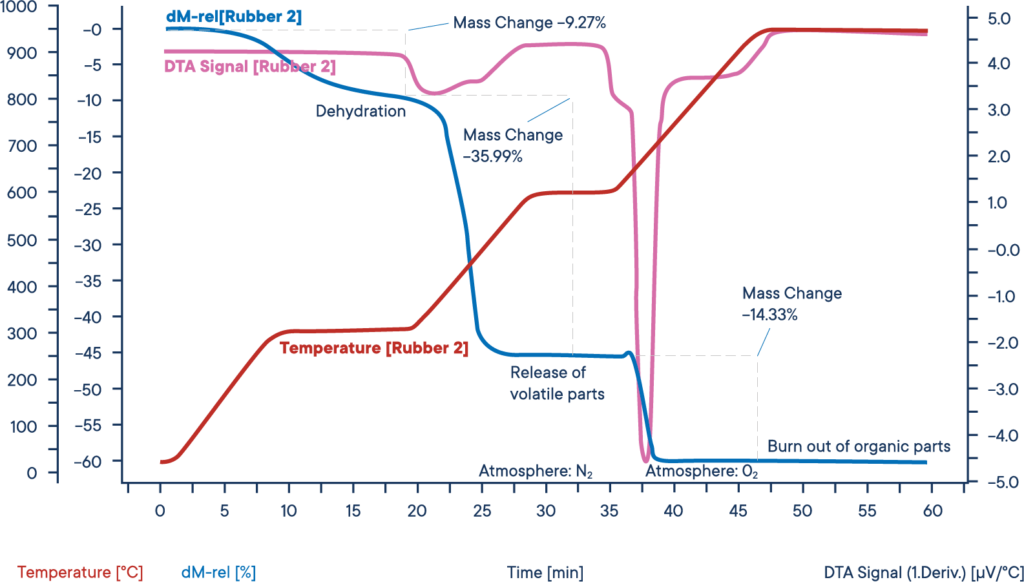
Decomposition of rubber in a STA (TG+DSC), pink curve is the DTA/DSC signal, blue curve is the mass loss
In addition, the interpretation of the measured thermal effects is facilitated and conversion enthalpies can be corrected for weight changes.
Furthermore, Simultaneous Thermal Analysis compensates for the uncertainties of separate TG and DTA/DSC measurements caused by sample inhomogeneities, sample geometry and temperature inaccuracies.
A clear assignment of TG and DTA/DSC results allows an almost complete sample characterization, especially for complex reactions. The complimentary information obtained allows differentiation between endothermic and exothermic events, which have no associated weight change (e.g. melting and crystallization) and those which involve a weight change (e.g. degradation).
