Description
To the point
The Fourier transformed infrared spectroscopy (FTIR) is a technique which is used to obtain an infrared spectrum of absorption of a solid, liquid or gas. Due to specific interactions of molecular bonds with infrared light, this absorption spectra can identify multiple compound classes. A FTIR spectrometer simultaneously collects spectral data in a wide spectral range, typically between 100 to 10000 cm-1. This confers a significant advantage over a dispersive spectrometer which measures intensity over a narrow range of wavelengths at a time. FTIR has made dispersive infrared spectrometers all but obsolete, opening up new applications of infrared spectroscopy.
The combination of a Linseis Thermal Analyzer with a FTIR is especially interesting in fields such as polymers, chemical and pharmaceutical industry, mainly if organic components are involved. The coupling is more than the sum of the separate parts. Benefits come from the LINSEIS coupling knowledge and integrated hard- and software concept. For interpretation of the result, different libraries are available.
Description
Both manufacturers Linseis and ThermoFisher Nicolet are some of the leading companies in their specialized fields. This cooperation guarantees outstanding performance for the combined system.
- Transfer line is temperature controlled up to a maximum of 250°C.
- Fast response time through short transfer line.
- FTIR detector with very high sensitivity and with different wave numbers available.
Coupling the measurement methods opens up a wide range of possible applications
- Outgassing of materials
- Analysis of additives, aging and decomposition processes
- Absorption and desorption behavior
- Natural and raw material characterization
Properties
- Top-shell research balance (various models); TG or STA (TG+DTA/DSC)
- Precision Nicolet FT-IR Spectrometer (different models available)
- Three separate heating zones: the capillary; the protection tube and the FTIR measuring cell
- Low purge gas flow rates
- Specially developed JLF detector with a very long optical absorption path

Unique Features

Combined thermogravimetry and FTIR:
Provides insightful information on material
outgassing and chemical composition.
High detection sensitivity:
heated capillary transfer path up to 300 °C.
Precision Nicolet
FTIR spectrometer:
Various models available.
Heated transferline:
Heating up to 250°C to ensure optimum substance transfer into the measurement cell.
Questions? We're just a call away!
+1 (609) 223 2070
+49 (0) 9287/880 0
Our service is available Monday to
Thursday from 8-16 o’clock
and Friday from 8-12 o’clock.
We are here for you
Specifications
Hard Facts
Thermobalance + FTIR Spectrometer in the temperature range from -170°C up to 1750°C
- L81/I-FTIR Thermo balance + FTIR Spectrometer
- L81/II-FTIR Thermo balance + FTIR Spectrometer
- STA L81-FTIR Thermo balance + FTIR Spectrometer
- STA L81-FTIR Thermo balance + FTIR Spectrometer
MODEL | FTIR - GAS ANALYSIS (EGA COUPLING) |
|---|---|
| Wavenumber range: | 7500 cm-1 … 370 cm-1 |
| Resolution: | 1 cm-1 |
| Heating: | Transfer line and adapter |
| Transfer line material: | PTFE (interchangeable) |
| Couplings: | DIL, TMA, STA, TGA, DTA, DSC |
Applications
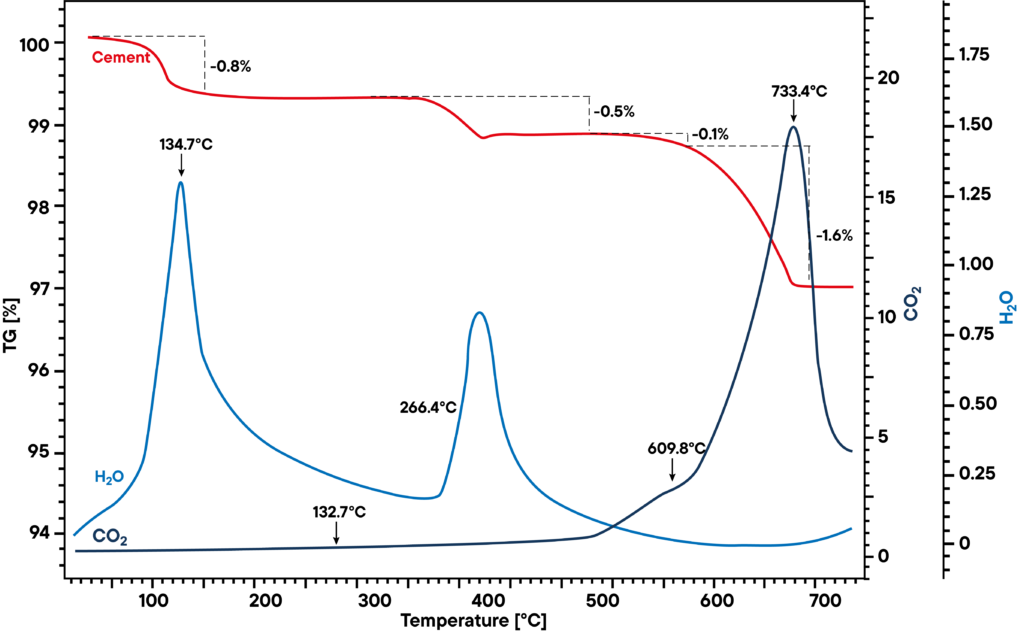
Applications example: Cement
Cement is an inorganic, non-metallic material. Together with water it hardens and afterwards it stays also hard under water. Portland cement e.g. consists of limestone, clay and/or sand. The additives gypsum, various anhydrites etc. influence the setting time of the cement. Impurities in the raw cement cause negative influence on the quality of the cement.
Analysis using thermogravimetry and FTIR
The additives of a cement sample can be identified and quantified by thermal analysis. The measurement on the right shows thermal analysis of a cement sample.
The red TG-curve shows a three-step decomposition where the first effect is the evolved water from the CaSO4 di-hydrate to CaSO4 half-hydrate. The second step is the conversion of the CaSO4 half-hydrate to the CaSO4 anhydrite. The evolved water can also be verified by FTIR analysis. Between 600°C and 750°C the carbonates decomposed so COs2 evolves. The shoulder in the CO2 trace of the FTIR signal is the decomposition of MgCO3.
Well informed

