The chemical formula of gypsum is CaSO4 ∙ 2H2O (calcium sulphate dihydrate). There are a variety of uses for gypsum as a building material. Gypsum is a dry powder that is mixed with water to form a paste, which then hardens. It remains fairly soft after drying and can be easily worked with metal tools.
Calcium sulphate hemihydrate (CaSO4 ∙ ½H2O) reacts with water to form calcium sulphate dihydrate and, when heated, calcium sulphate dihydrate separates water and exchanges it for calcium sulphate hemihydrate. The cleavage of CaSO4 ∙ 2H2O crystalline is caused by CaSO4 bilayers (in each layer Ca2 + and SO4 2 ionics are alternately next to each other) with relatively low hydrogen bonds.
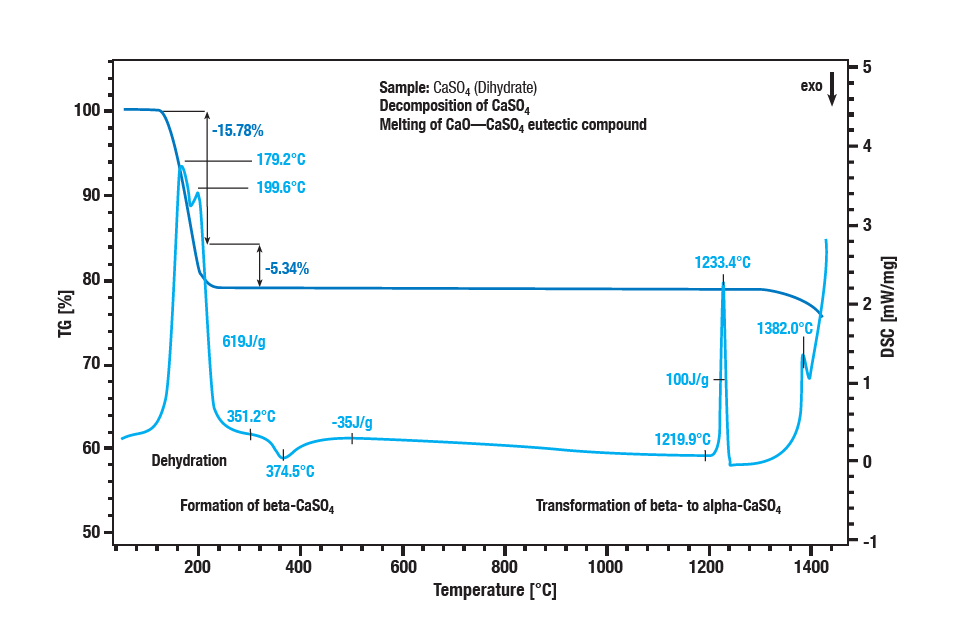
The two-stage dehydration of calcium sulphate dihydrate occurs between 100 °C and 300 °C. The first step is the formation of hemihydrate (CaSO4 ∙ 2H2O to CaSO4 ∙ ½H2O). A further dehydration forms the anhydrate (CaSO4 ½H2O to CaSO 4). With an exothermic effect at approx. 340 °C, the anhydrate converts to β-calcium sulphate. The exothermic effect in the curve at approx. 1220 °C is the conversion of β-calcium sulphate into α-calcium sulphate. Sulphate decomposition is characterised by a further loss of mass at temperatures above 1250 °C. Calcium sulphate is converted into calcium oxide. The melting of a eutectic mixture of calcium sulphate and calcium oxide is the peak at 1380 °C.
