Graphite is a carbon species that occurs as a dark grey solid. It has a considerable high chemical resistance and is used in many ways, for example as cathode material, construction material, sensor component and many more.
If heated, it reacts with oxygen to carbon monoxide or carbon dioxide, however it can reach very high temperatures if it is heated in inert, oxygen free environment and for this reason it is used in ultra-high temperature furnaces as furnace material or even heater.
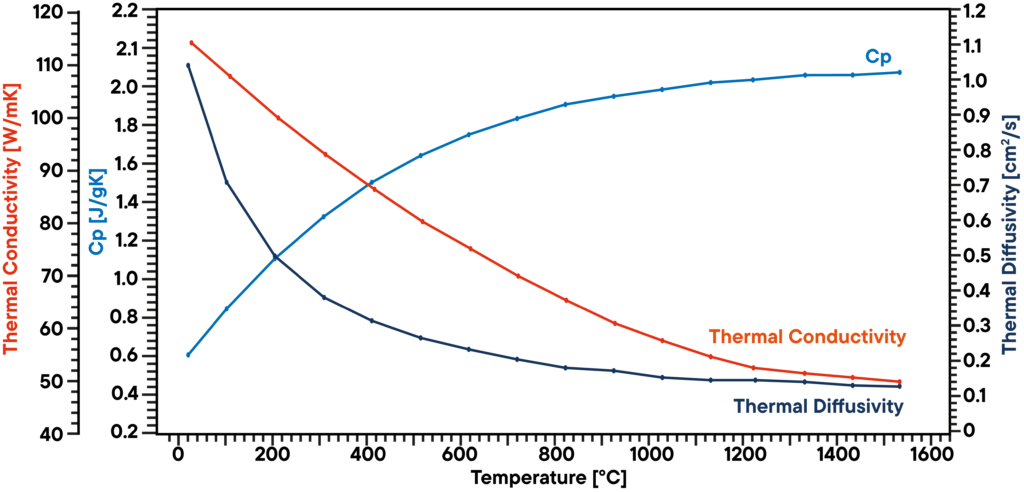
In this example, a graphite sample has been analyzed in vacuum using a LFA 1000 (Laserflash Analyzer). Thermal diffusivity has been measured directly at several temperature steps between RT and 1100°C.
Specific heat capacity has been determined using a known graphite standard in a second sample position as a reference in the same measurement. The product of diffusivity, specific heat and density gives the corresponding thermal conductivity. The result shows a continously decreasing thermal conductivity which is typical and a thermal diffusivity that is showing a plateau above 500°C. The Cp is increasing over temperature.

