Phase Change Materials (PCMs), also called latent heat storage materials, are capable to store and release thermal energy during the process of a phase change (e.g. solid – liquid).
During freezing, the material releases large amounts of energy in form of latent heat of fusion and when the material is melted, an equal amount of energy is absorbed from the vicinity. By that PCMs can be used for heating or cooling. The simplest example for a phase change material is water/ice. Also, various paraffins and salt hydrates belong to the PCMs.
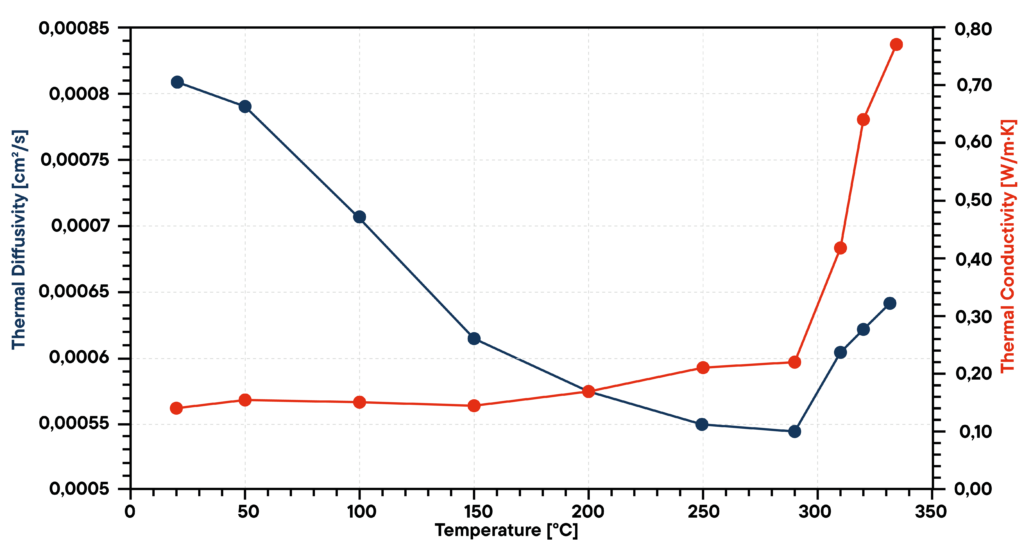
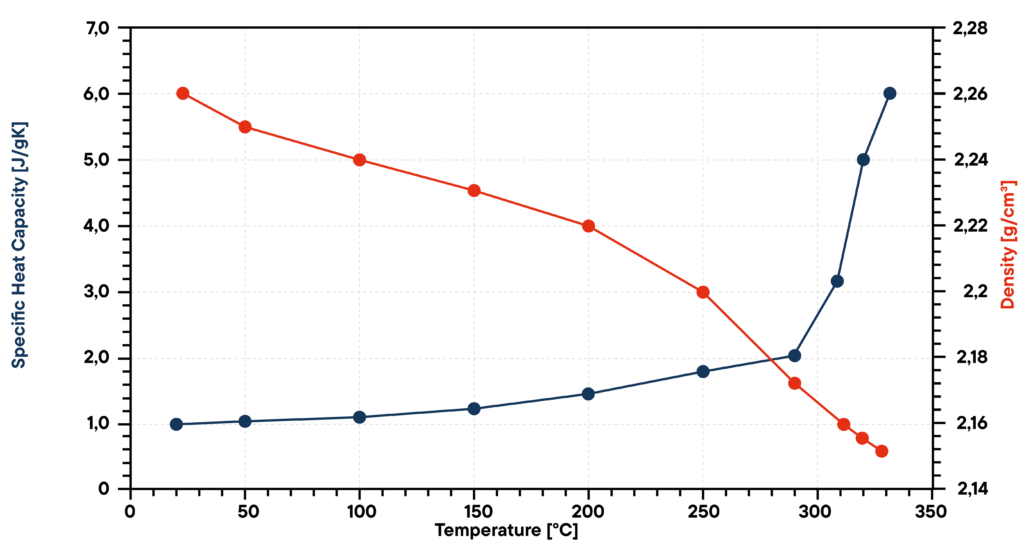
In this case sodium nitrate (NaNO3) was investigated from room temperature to 330 °C. The thermal diffusivity was measured by LFA, the specific heat capacity by DSC and the density by a dilatometer. With these parameters it was possible to calculate the thermal conductivity.
The phase change from solid to liquid at a temperature of about 310 °C is visible in all graphs. The thermal diffusivity of the solid decreases with temperature and in the liquid state starting from 310 °C the diffusivity increases. The specific heat capacity increases rapidly after the phase change. With increasing temperature, the density decreases. The thermal conductivity behaves similar to the specific heat capacity, it increases in the solid state slightly and in the liquid state more rapidly.