Carbon containing materials, organics and polymers usually burn off when heated. The investigation of thermal decomposition of such materials is therefore a bit special. In most cases it is carried out in inert atmospheres instead of air to be able to see decomposition effects and pyrolysis, followed by a gas switch to oxygen or air, leading to a burn off of the contained carbon.
If this procedure is carried out on a combined thermal analyzer (STA), the carbon content, inorganic content and released heat can be measured.
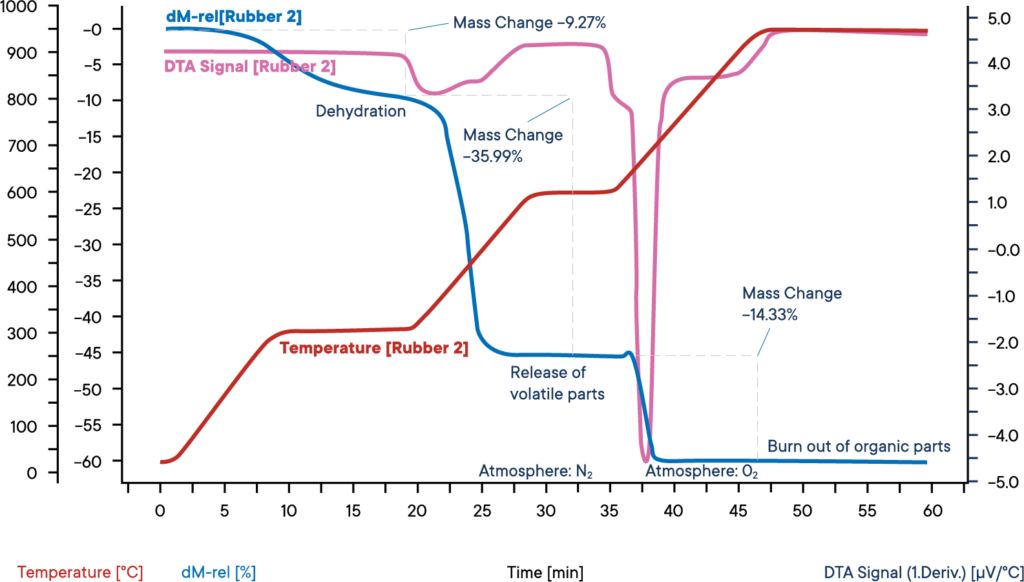
This measurement of an industrial rubber sample was carried out with a simultaneous thermal analyzer STA L81, starting at nitrogen atmosphere. The sample was heated in three steps with each 30 K/min.
The blue curve shows the relative weight loss. In a first weight loss step, the dehydration of the sample takes place. The amount of water was 9.3 %. The corresponding DTA signal (purple curve) did not show any effect during the evaporation of water.
In the second reaction step, the volatile components are released by pyrolysis un- der N2 atmosphere. The amount of these components is 36.0%. Their release can be identified by an exdothermic reaction peak on the DTA curve. For the third reaction step, the atmosphere is changed to O2, leading to a burn off of the remaining carbon.
The loss in weight is 14.3 %. The remaining 40.4 % are inorganic components like ashes, slake or fillers.


