The Linseis STA HP series allows measurements under controlled elevated pressure. For some reactions like decompositions, adsorption and desorption, the behavior of samples and materials is very much depending on the atmospheric conditions as there is a pressure dependency of many reactions.
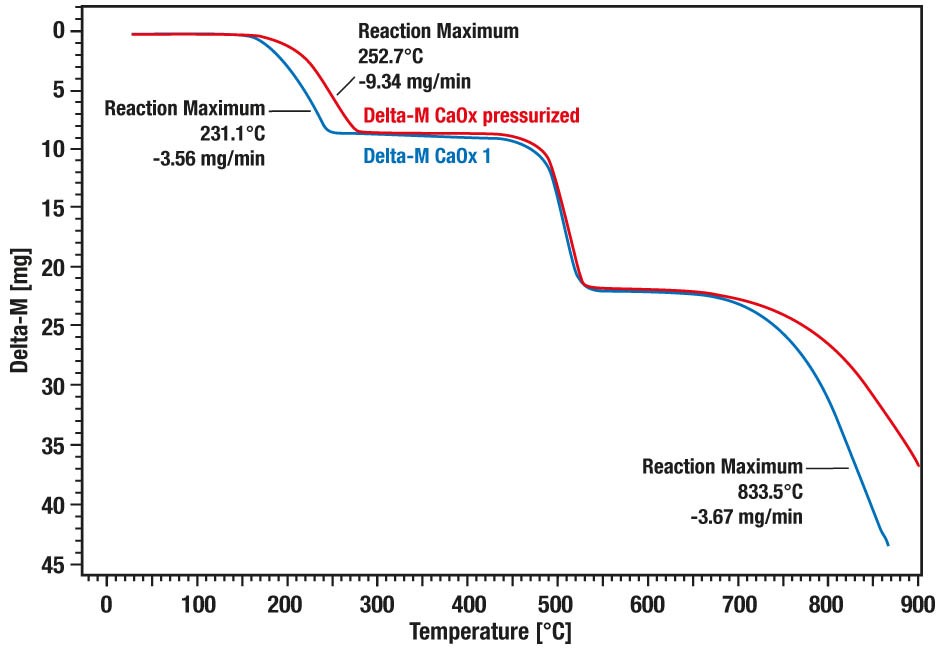
These curves show the comparative measurement of calcium oxalate hydrate decomposition under pressure (20 bar, red curve) vs. atmospheric condition (blue curve). A significant pressure dependence of the decomposition steps 1 (loss of water) and 3 (loss of carbon dioxide) can be observed. The decomposition steps 1 and 3 are shifted to higher temperatures at elevated pressure. The second step is the irreversible transformation from organic oxalate to inorganic carbonate, releasing carbon monoxide. As this is not reversible it is not pressure depending.

