A typical application for high pressure TGA measurements is the investigation of the so-called coal gasification or hydro-gasification. This process, where carbon is heated in a water steam atmosphere, is used in catalytical processes, for example to remove carbon monoxide from exhaust fumes and especially to get valuable organic compounds out from resources like charcoal or biomass.
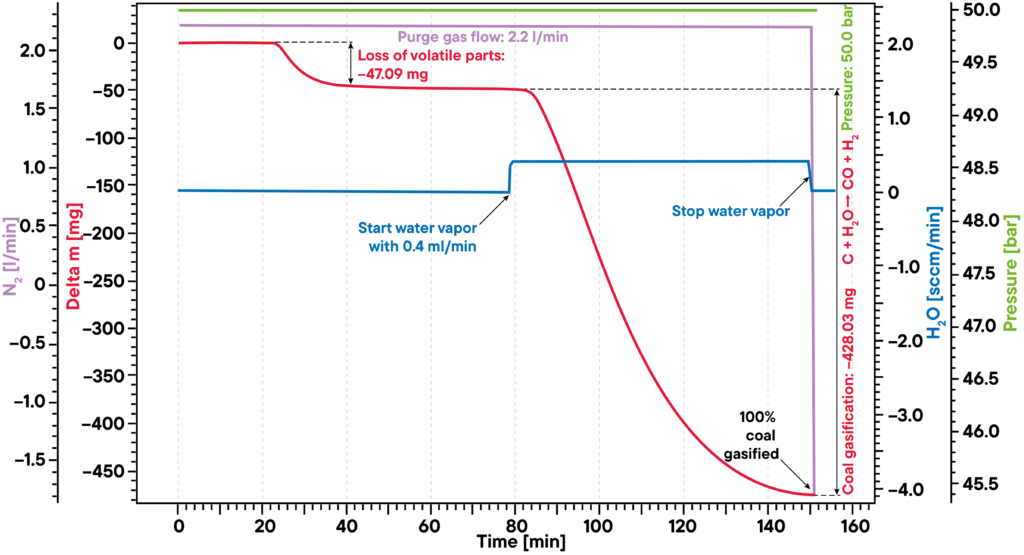
The given example shows a typical gasification experiment of charcoal. The coal sample was heated to an isothermal plateau under nitrogen atmosphere at 50 bar pressure (High Pressure TGA – Thermobalance). The mass signal shows the loss of volatile components between 20 and 40 min. After water vapor was added, the coal was gasified and nearly completely consumed after 150min, leading to H2, CO, CH3OH and other useful reactive gases, as shown by the red mass loss curve.
The whole process can be described like this: Carbon reacts with water vapour to a mixture of carbon monoxide and hydrogen. The obtained carbon monoxide can react with a second water molecule to carbon dioxide and additional hydrogen and finally the resulting hydrogen can form methane and other hydrocarbons out of carbon monoxide.

