Titanium hydride is a common used hydrogen resource for controlled release of hydrogen in various reactions. On the one hand it can be used as a catalyst in liquid chemistry in situ as a hydrogen source, on the other hand it can be used for example in batteries or fuel cells for controlled hydrogen release.
To get an idea what amount of hydrogen is released at what temperature, it is important to know the temperature dependent decomposition behavior and released amount of heat, which can be monitored by simultaneous thermal analysis (STA).
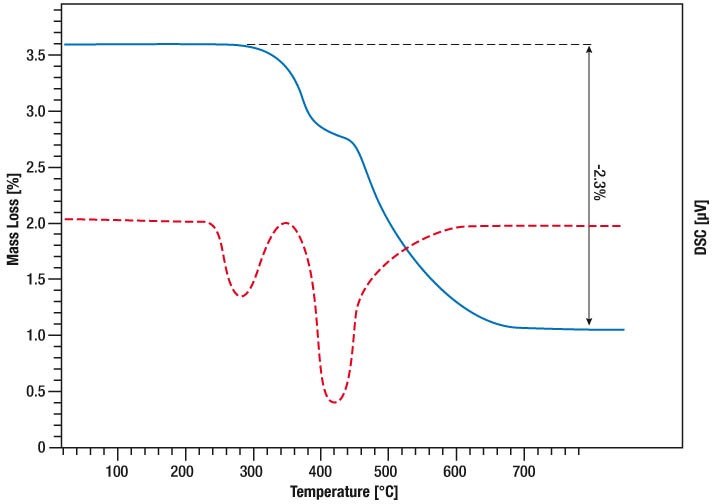
In this STA measurement, the release of hydrogen by titanium hydride was monitored. TG and DSC signal were measured from Room Temperature to 800°C while the sample was heated linear in Argon atmosphere with 10K/min. Between 300°C and 600°C, there is a two-step mass loss of 2.3% in total which means the complete amount of bound hydrogen is released in that process. The DSC curve shows the corresponding Desorption peaks (red curve).

