Enthalpy
Enthalpy – a measured variable in thermal analysis
What is enthalpy?
The reaction enthalpy is the enthalpy change of a sample during a chemical reaction. Reactions that release energy in the form of heat are called exothermic. Reactions in which energy must be supplied as endothermic.
Melt enthalpies are a simple example of endothermic processes, since one usually has to give heat work in a system in order to break up its solid crystal structure and convert it into a liquid phase with molecules that move freely relative to one another. An example of an exothermic reaction is a simple combustion process in which a substance reacts with oxygen to release energy.
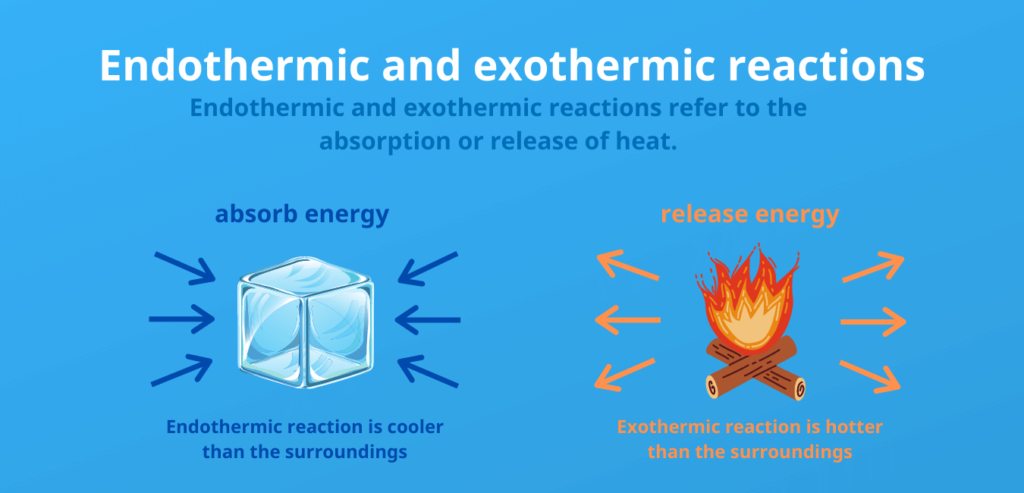
The difference between endothermic and exothermic release:
Endothermic:
- Energy is consumed (heat is absorbed)
- Energy is consumed or needed for reaction to occur
- Increase of inner enthalphy
Examples of endothermic processes include:
- Dissolving salts in solvent
- Cracking alkanes
- Evaporating liquids
- Melting solids
Exothermic:
- Energy is released (heat is released)
- Reaction occurs spontaneously or triggered
- Decrease of inner enthalphy
Examples of exothermic processes include:
- The thermite reaction
- A neutralization (e.g., mixing an acid and a base to form a salt and water)
- Most polymerization reactions
- Combustion
- Respiration
- Corrosion of metals (an oxidation reaction)
- Most crystallization processes
