Table of Contents
Crystallization describes the transition of a substance into a crystalline state. This can occur from the gas phase, solid phase or liquid phase. Atoms or molecules arrange themselves into a clearly defined crystal lattice with minimum energy states.
Scientific definition
Crystallization, both in metallurgy, glass ceramics and polymers, is the process in the cooling process where crystallites form, either by themselves or by added nucleating agents and impurities.
Here, due to the main application of DSC, crystallization in polymers that form a crystal structure through regions of long-range order. Previously, these molecular chains existed in a disordered amorphous state.
In addition to the crystallization nuclei, the prerequisite for crystallization is a melt and, consequently, a sufficiently high temperature. The slower the cooling, the better the crystallization can be detected. This gives the corresponding molecular chains more time to orient themselves.
Recrystallization
However, crystallization can also be caused by stretching due to mechanical influences in a cold state. On reheating, recrystallization can occur. This recrystallization, also called cold crystallization, occurs between the glass transition temperature and the melting of the polymer.
The cause of this exothermic reaction is the further orientation of the chains as a result of the softening of the material after the glass transition point. The softening allows the molecular chains to vibrate better and subsequently orient themselves to form crystals. In the process, new crystallization nuclei can be formed as well as existing crystal grains can grow.
Practical application
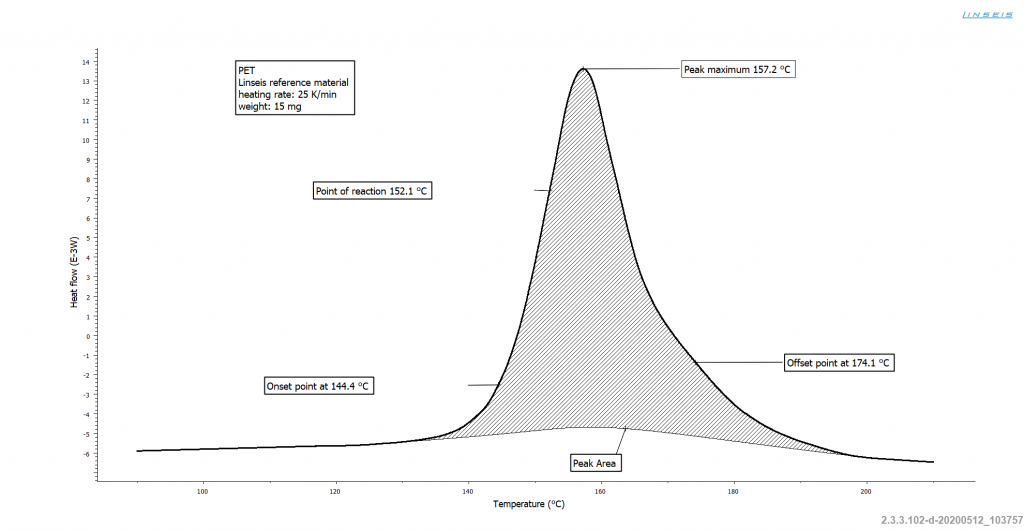
In daily practice of DSC measurement, crystallization manifests itself as an exothermic peak. Crystallinity, degree of crystallization and degree of crystallinity are used synonymously in the technical literature and indicate the percentage of the polymer that is crystalline.
In practice, completely crystalline polymers are of little importance, since they can only be produced by elaborate annealing just below the melting temperature.

Determination of crystallinity
K = degree of crystallinity
∆H = enthalpy
∆HC = enthalpy of crystallization
∆Hlit = literature value of enthalpy
∆HM = enthalpy of melt
A prerequisite for determining the degree of crystallinity is knowledge of the theoretical enthalpy of fusion ∆Hlit for completely crystalline material, according to:
K=∆HM/∆Hlit ∙100
the degree of crystallinity can be calculated in percent. By heating the sample for a second time, it can thus be determined to what extent the crystallinity for this sample was influenced by the thermal and mechanical history. If a recrystallization peak occurs during the heating process, it is subtracted from the enthalpy of fusion and the result is divided by the associated literature value. This can be summarized by:
K=(∆HM–∆HC)/∆Hlit ∙100