Table of Contents
Scientific definition of Raman spectroscopy
Raman spectroscopy is a technique for studying molecules and determining their structure and dynamics. It uses excitation-induced scattering of light to study chemical bonds in a sample. The technique is useful for understanding structures and dynamics of molecules in the solid, liquid and gas phases.
What is Raman spectroscopy?
Raman is a technique that allows for the determination of molecules and molecular bondings. This type of spectroscopy is particularly helpful as it enables a direct measurement of chemical composition without the need to break down a sample. Raman spectroscopy is a non-invasive technique that offers high accuracy and repeatability. The precise and rapid analytical measurement allows scientists and laboratory experts to analyze a wide range of substances quickly and efficiently, thereby determining the chemical composition of a sample rapidly and accurately. Thanks to this measurement method, qualitative and quantitative analyses can be conducted in research, industrial applications, and medical diagnostics, among other areas. It is also highly useful for supporting the stability of materials, process monitoring, quality control, and sample identification.

A Raman spectrometer measures the Raman scattered light that occurs during the interaction of light with a material. This scattering changes the wavelength of the incident light and provides information about the chemical bonds within a material.
Fields of application of Raman spectroscopy
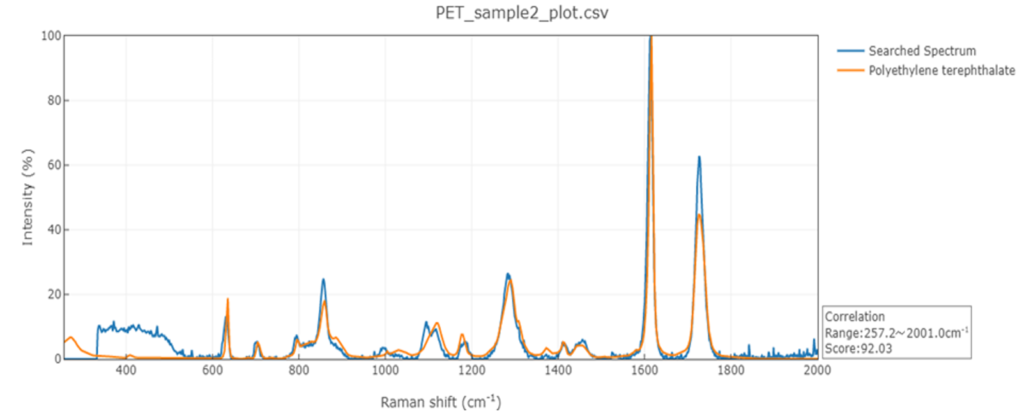
Raman spectroscopy can be used to analyze a wide range of materials, such as organic compounds, polymers, and specific minerals. Raman spectroscopy is particularly useful for examining samples that provide only limited information, as the technique not only provides information about the chemical structure but also the spatial arrangement of molecules (i.e., crystal structure). Another advantage is that Raman spectroscopy is highly sensitive and can detect even small changes in sample molecules. The technique can also be employed to measure impurities and trace substances.
Raman spectroscopy can measure various molecule-to-molecule bonds, such as:
- C-C (carbon-carbon) bonds in organic compounds
- C-O (carbon-oxygen) bonds in carbonyl groups
- N-H (nitrogen-hydrogen) bonds in amides
- S-O (sulfur-oxygen) bonds in thiols
- Quality control in the pharmaceutical and chemical industries
- Identification of materials in archaeology, art history, and forensics
- Analysis of solids and liquids in materials science
- Monitoring processes in energy and environmental engineering
- Examination of biological samples in life science research.
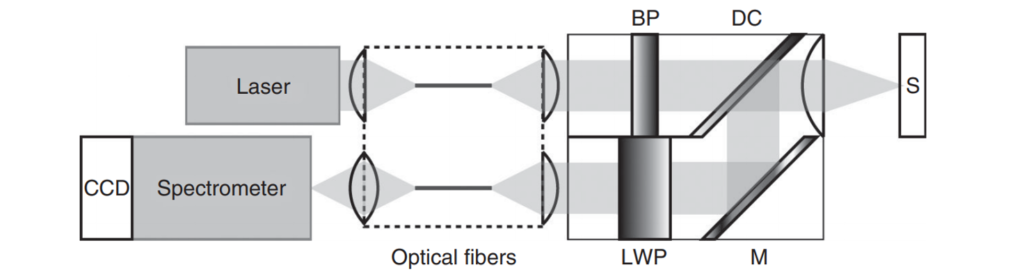
Construction of a Raman spectrometer
A Raman spectrometer consists of the following main components:
- Light Source:
- provides the incident light, usually a laser system.
- Optical Components:
- such as lenses and mirrors, to direct the light onto the sample material and collect the Raman scattered light.
- Sample Holder:
- holds the material to be examined.
- Detector:
- measures the scattered light emitted by the sample and converts it into electrical signals.
- Electronic Components:
- such as amplifiers and analyzers, to process the signals and produce the Raman spectra
Combination option: DSC and Raman spectrometer
Thanks to more efficient data acquisition and miniaturization, Raman spectrometers have become much more affordable. For this reason, combining this method with other means has become increasingly economical in recent years.
For instance, a Raman spectrometer can be coupled with a DSC (Differential Scanning Calorimeter). In this way, both the enthalpic effects of a sample measurement can be quantitatively represented and the Raman spectrum can be recorded simultaneously, allowing for statements about molecular chain bonds and crystallinity, among other things.
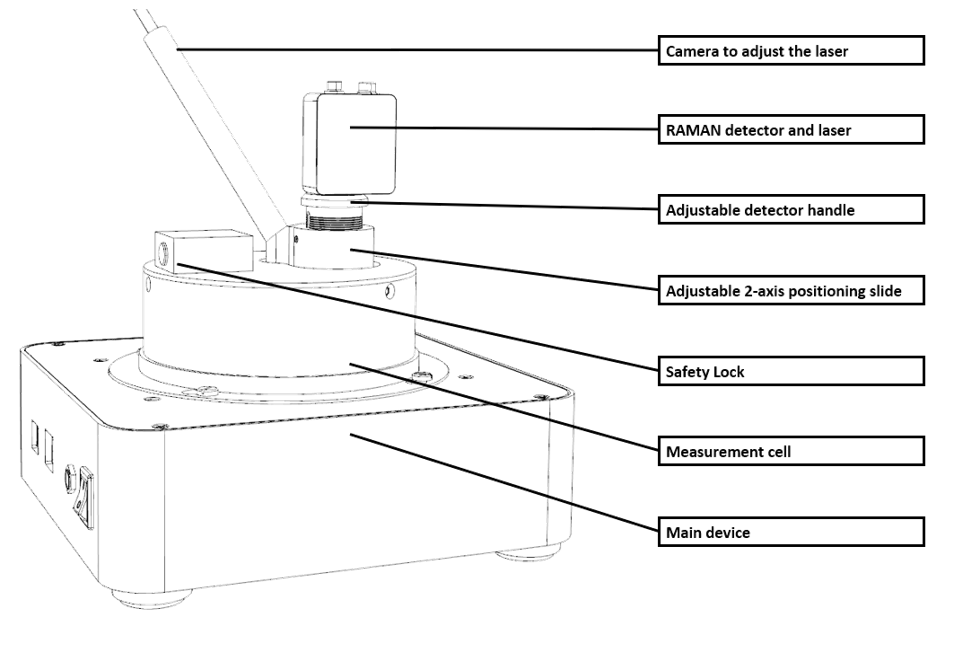
Applications
This can be advantageous in a variety of applications in material and process development, such as in the characterization of polymers, solids, battery materials, and biological samples.
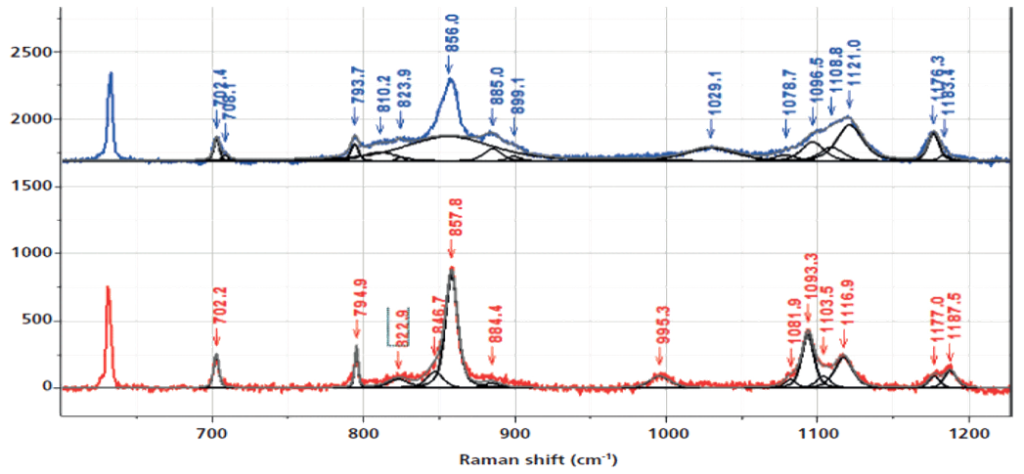
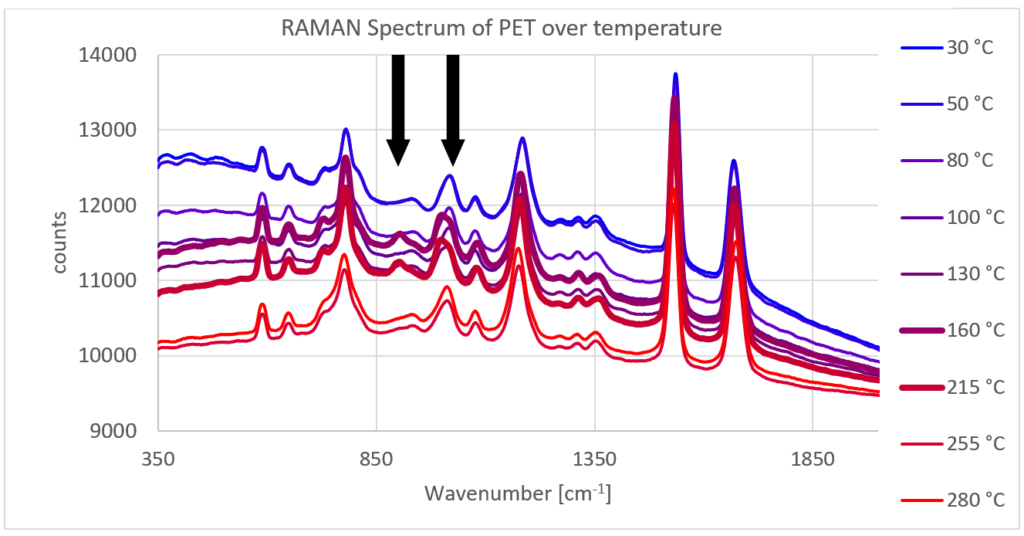
For example, a simple heating process of a PET (Polyethylene Terephthalate) sample reveals various thermal effects such as a glass transition (~80 °C), recrystallization (~150 °C), and the melting of the sample (~250 °C).
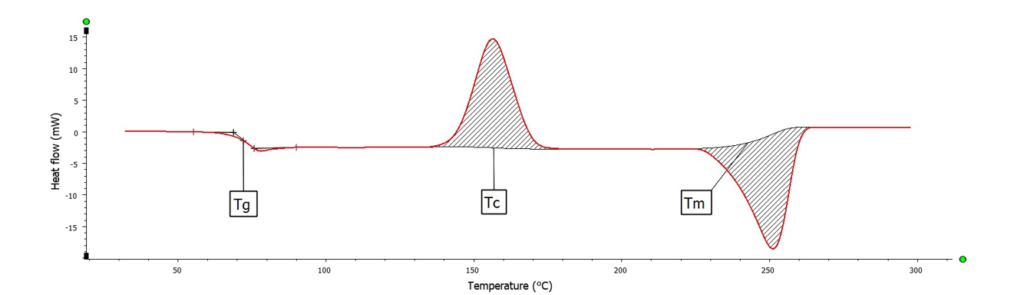
Using Raman spectroscopy, the origin of these effects can be traced back based on the Raman spectrum, for example, through the crystallinity: